
FDA Approves Second COVID Booster For Adults Above 50 - When Will India Follow?
At least 36 countries have approved booster doses for their citizens. When will India follow suit?
advertisement
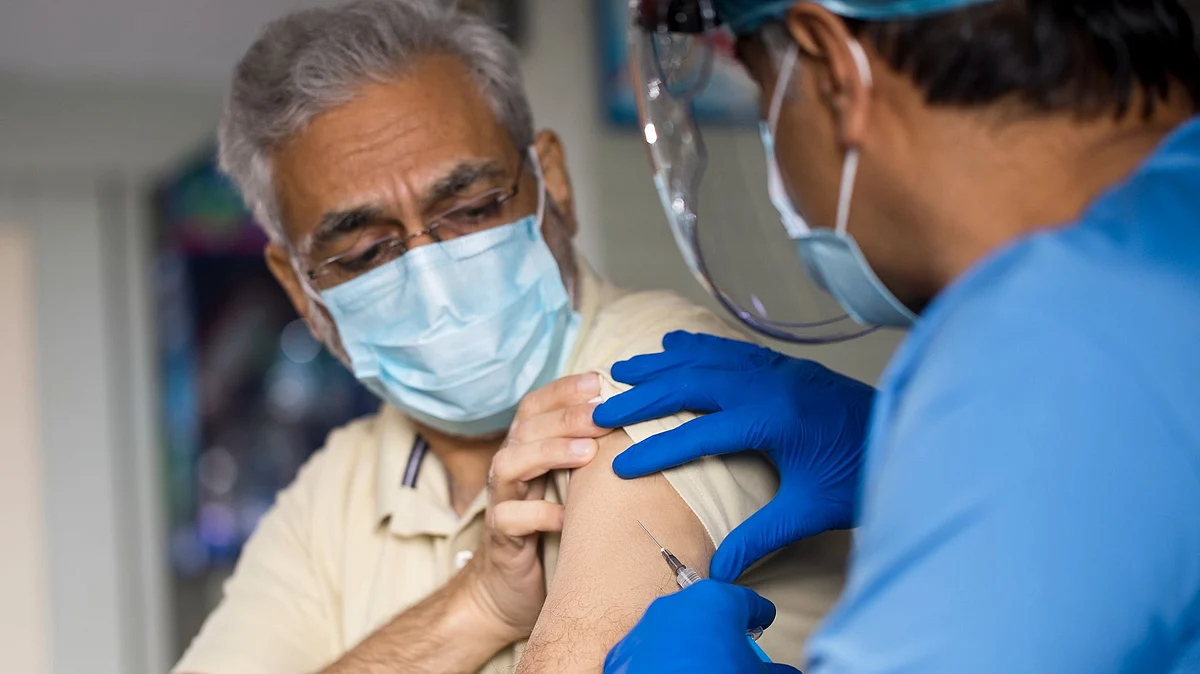
The Food and Drug Administration (FDA) approved a second booster dose of the Pfizer-BioNtech and Moderna COVID-19 vaccines for older adults (50 and older), on Tuesday, 29 March 2022.
The FDA also updated its authorization of additional doses for people 12 and older who are immunocompromised, saying they are eligible for another booster shot, following a recommendation from the Centers for Disease Control and Prevention.
In November 2021, the FDA had approved the administration of a third dose of the COVID-19 vaccine for adults aged 50 and older.
In December 2021, the FDA also approved a third vaccine dose i.e., the first booster shot for children aged 16-17 and those at higher risk, like the immuno-compromised.
The second booster can be administered four months after the first booster dose, according to the CDC.
When Will India Authorize Vaccine Booster Doses?
While India is yet to roll out booster doses for all its citizens, in December 2021, the Centre announced that it would administer "precautionary doses" to frontline workers, healthcare workers, and those above 60 at risk.
The statement said that frontline workers and healthcare workers could avail the booster dose from 10 January 2022.
In January 2022, The Drugs Controller General of India (DCGI) also gave permission to Bharat Biotech to commence intranasal COVID-19 booster shot trials.
In March 2022, a study submitted to the DCGI showed that administering a booster dose of the Covishield vaccine after two doses of Covaxin was extremely effective in improving antibody response.
India's COVID-19 booster response has fallen behind drastically when compared to other countries. As many as 36 countries have authorized booster doses of the vaccine including the US, the UK, Turkey, France, Chile, and many more.
However, India is yet to receive an official announcement or authorization of a booster dose for all citizens.
- Access to all paywalled content on site
- Ad-free experience across The Quint
- Early previews of our Special Projects
Published: undefined